Centre for Self-Assembled Chemical Structures (CSACS)
The CSACS includes researchers from 5 Quebec universities. In addition to the Université de Montréal, McGill University, Concordia University, the Université de Sherbrooke and the Institut national de recherche scientifique (INRS) are all represented.
What is self-assembly?
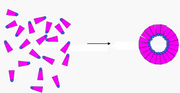
Self-assembly refers to phenomena whereby small chemical units are introduced into an environment, reach equilibrium state, and produce much larger-scale structures and patterns. Self-assembly strongly mimics natural mechanisms that form stable and complex architectures from simple building blocks.
- Liquid crystals
- Self-assembled monolayers
- Organization of lipids into micelles and membrane vesicles
- Block copolymer microphase separation
- Polymers adsorbed onto surfaces in layers and multilayers
Self-assembled monolayers
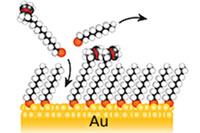
Organic self-assembled monolayers are a widely used method for surface modification of metals and metal oxides in fundamental investigations of interfacial phenomena and design of molecular-scale devices.
Centre members are designing new functionalized monolayers for applications in catalysis by incorporating organometallic groups, in optoelectronics by creating films with extended conjugation, and for model biological surfaces and biosensors by adding molecular recognition groups.
Molecular-level characterization of these films is critical to their planned applications. Novel characterization techniques employed by the Centre members include:
- Electrochemical-atomic force microscopy (EC-AFM)
- Surface plasmon resonance spectroscopy (SPR)
- Microcantilever sensors
- High resolution solid-state NMR
In addition they use a spectroscopic method that probes the chemical and physical modifications induced in thin organic films by very low energy electron impact.
Patterned self-assembled films
Phase-separation in mixed monolayers of phospholipids, alkylthiols, and alkylsilanes is being exploited to create self-patterned monolayer assemblies that are chemically or physically differentiated. These thin films are used to template the selective deposition of macromolecules, nanoparticles, and the crystallization of organic molecules on the submicron scale, thereby creating new surface structures and materials.
Organometallic thin films
Metal ions can organize organic fragments (ligands) in a predetermined manner to dictate the form and function of the final assembled structure. The design and self-assembly of discrete multi-component structures rely on recognition between the constituent building blocks. The supramolecular chemistry and self-assembly of these molecules at surfaces are being investigated with:
- Atomic force microscopy (AFM)
- Scanning tunnelling microscopy (STM)
- Transmission electron microscopy (TEM)
Synthesis, characterization and self-Assembly of photon- and electron-sensitive molecules
The target species being synthesized consist of hydrophilic polymer backbones supporting high-density azobenzene side-groups and termination motifs including NO2 and CN. These materials are deposited as oriented monolayers on Au/mica supports and characterized using AFM and grazing incidence infrared spectroscopy; trans/cis isomerizations are induced by optical irradiation in the UV/vis regions.
Organic layers
Despite the wide use of graphite electrodes in electrochemistry, there have been few investigations of their surface and electrochemical properties. The factors influencing the efficiency, stability, and selectivity of graphite electrodes modified with aryl layers are analyzed in certain electrochemical processes.
Biomimetic interfaces

The formation of complex macroscopic structures from component building blocks remains one of the most compelling problems in biology. This self-ordering process lies at the heart of how a complex function, such as cell mobility, can result in the structural and energy conversion of proteins.
However, there are not yet rules that predict the eventual structure or pattern formed by a self-organization process from either mechanism. Only through a detailed understanding of the kinetics and energetics of the self-assembly/self-organization sequence of model systems will it be possible to formulate such rules. The research in this area focuses on the 2-dimensional organization of lipids and proteins at surfaces. Libraries of bi-and tri-polar lipids have been created and their self-assembly are studied to construct structure-property phase diagrams.
Phase transitions in lipid monolayers and bilayers
The spatial organization of proteins, lipids, and guest molecules in lipid monolayers, membranes, and thin films ultimately determines their specific interactions and function. The spatial resolution of AFM and LFM (lateral-force microscopy) are used to investigate mixing in phospholipid monolayers and bilayers formed by the Langmuir-Blodgett technique or by vesicle fusion onto mica.
Defects and pore formation in membranes by amphipathic peptides
Organic synthesis is being used to access new materials that integrate the structural and functional characteristics of biopolymers with the stability and diversity of synthetic polymers. The result of this research collaboration is improved understanding of synthetic macrocycle and oligomer self-assembly within model membranes. In particular, the channel- or pore-forming nature of synthetic foldamers and macrocycles is being studied using AFM.
Polymer self-assembly

Van der Waals forces, hydrophobic and electrostatic interactions are used to guide the 3rd-order structure of synthetic polymers into an impressive range of morphologies.
Copolymers with subunits designed to drive the self-assembly via molecular recognition, hydrophobic interactions and electrostatic interactions are being designed by Centre researchers.
Micelles of small molecules are incorporated into polymers, and then easily removed, to fabricate porous polymers. Functionalized polymer films are created through Langmuir-Blodgett techniques and by the layer-by-layer polyelectrolyte method.
Identification of the resulting structures relies on the combined efforts of the Centre's characterization experts, allowing the exploration of possible applications as biosensors, materials for bioseparations and drug delivery agents.
Self-assembly of polymers via molecular recognition
A novel approach is to synthesize polymers that are able to use molecular recognition to self-assemble into higher-order morphologies, in the same manner that selective base-pairing organizes DNA into a double helical molecule.
Advanced NMR and vibrational spectroscopic methods are used to characterize the hydrogen bonding interactions. The morphologies are characterized by light scattering and transmission electron microscopy.
Self-assembly of block copolymers via hydrophobic and hydrophilic interactions
Considerable effort has been devoted to the study of hydrophobically-modified polymers (HM-polymers), in view of their theoretical relevance and practical applications.
Such polymers consist of a water-soluble chain carrying a few hydrophobic groups, hydrocarbon or fluorocarbon chains. It is well known that fluorocarbons and hydrocarbons do not mix. The question then arises: what will be the properties of HM-polymers carrying both hydrocarbon and fluorocarbon chains?
Optically active self-assembled block copolymers
The synthesis of block copolymers containing photo-switchable groups allows one to address their self-assembled structures for reversible changes in optical, geometric or mechanical properties. Azobenzene derivatized block copolymer vesicles, photoactive elastomers and optically active polymer films are produced by several groups and the Centre's researchers also have the optical expertise to examine the functionality of these photo-addressable self-assembled polymeric materials.
Block copolymer Langmuir-Blodgett films
The spontaneous phase separation of AB diblock copolymers can be exploited for the preparation of patterned surfaces exhibiting periodically ordered domains of molecular dimension. Such surfaces can serve as templates for directed cell growth. A significant limitation to this approach is the general lack of control over microdomain orientation. Films of asymmetric block copolymers are always polydomain with periodic ordering being maintained typically over a few microns.
Polyelectrolyte multilayers

The alternate adsorption of cationic and anionic polymers has become an increasingly important method for producing uniform thin polymer films. Recently, simple PEMs were deposited on silica colloids and high resolution solid-state NMR spectroscopy used to follow the layer-by-layer growth and polymer complexation. One of the goals of this research is to understand the effect of variation of the preparative parameters on the film properties at a molecular level.
Liquid crystalline materials
The liquid crystalline state is a fundamental state of matter that combines mobility with structure. Biological membranes function in this state, which is thus essential to life itself. In the laboratory this is achieved by the self-assembly of molecules, and is therefore a basic design element for obtaining materials with specific structures and properties.
The CSACS research projects in this area reflect the creativity with which liquid crystallinity can be exploited to develop new and useful materials.
Liquid crystal gels
Liquid crystal gels are low-molar-mass liquid crystals whose long-range orientation or textures are stabilized by a polymer network. Generally, the network is covalent since it is formed by polymerization of a reactive monomer dissolved in the liquid crystal host. The use of non-covalent, self-assembled networks is being explored for making liquid crystal gels, which when functionalized with photoresponsive groups, create dynamic functional materials that have potential applications in display technologies.
We are exploring the reversible trans-cis photoisomerization of azobenzene to add new functionalities to these materials. In particular, we are interested in using them as optical materials to record diffraction gratings that can be controlled either by an electric field or light.
Liquid crystal polymers
Novel LC polymers have been synthesized by classical and supramolecular chemistry. Supramolecular chemistry combines small molecule and polymer properties, and allows tailoring of material properties. Thus, complementary polymers and mesogens are self-assembled through non-covalent interactions (ionic, hydrogen-bonding, coordination).
The use of block copolymers leads to hierarchical structures, combining the (block copolymer) mesoscale and (liquid crystal) nanoscale, that begin to resemble nature's structures. Stimuli-sensitive mesogens are chosen to generate intelligent materials for specific applications (for example, optical). These materials are being investigated in bulk, and as thin and ultra-thin films. Theory and computational modeling aid in understanding the various self-assembling processes involved.
Liquid Crystalline Suspensions
Under suitable conditions, rod-like colloidal particles can form liquid crystalline suspensions. When nanocrystals of cellulose are dispersed in water, they self-order to form chiral nematic phases with interesting physical and optical properties. Surface orientation of the nanocrystals, film roughness and stability to solvent were compared for the 2 multilayer preparation methods. Ionically crosslinked dip-coated films are stable in water whereas physisorbed spin-coated films re-disperse in solvent.
Attempts were made to induce alignment of the cellulose nanocrystals by varying the substrate, substrate pre-treatment, cellulose concentration and by applying a magnetic field.
CSACS equipment
- Instron tester
- Quartz microbalance
- Analytical and preparative GPCs
- DSC, TGA and DMTA
- SNOM
- Raman spectrometry
- Analytical ultracentrifuge
- WAXS and SAXS
- AFM microscopy
- Optical microscopy
- Static and dynamic light scattering
- Conventional FTIR
- Polarization modulation infrared FTIR spectrometer
- Accessories for IRRAS, single and multiple reflection ATR (attenuated total reflection)
- Surface force apparatus
- Transient fluorescence spectroscopy
- Contact angle apparatus
- Particle analyzer
- TEM and SEM
Information
Professor Géraldine Bazuin : 514 340-5176
Professor Robert Prud'homme : 514 340-5173
Infrastructure - Université de Montréal
- Materials Characterization Laboratory (LCM)
- Thin Film Research Laboratory (GCM)
- Regional Centre for Mass Spectrometry
- Elemental Analysis Laboratory
CSACS Professors
The CSACS includes 36 professors, 13 of them from the Université de Montréal.